Kras oncogene ablation prevents resistance in advanced lung adenocarcinomas
Salmón M, Álvarez-Díaz R, Fustero-Torre C, Brehey O, Lechuga CG, Sanclemente M, Fernández-García F, López-García A, Martín-Guijarro MC, Rodríguez-Perales S, Bousquet-Mur E, Morales-Cacho L, Mulero F, Al-Shahrour F, Martínez L, Domínguez O, Caleiras E, Ortega S, Guerra C, Musteanu M, Drosten M and Barbacid M.
KRASG12C inhibitors have revolutionized the clinical management of patients with KRASG12C-mutant lung adenocarcinoma. However, patient exposure to these inhibitors leads to the rapid onset of resistance. In this study, we have used genetically engineered mice to compare the therapeutic efficacy and the emergence of tumor resistance between genetic ablation of mutant Kras expression and pharmacological inhibition of oncogenic KRAS activity. Whereas Kras ablation induces massive tumor regression and prevents the appearance of resistant cells in vivo, treatment of KrasG12C/Trp53-driven lung adenocarcinomas with sotorasib, a selective KRASG12C inhibitor, caused a limited antitumor response similar to that observed in the clinic, including the rapid onset of resistance. Unlike in human tumors, we did not observe mutations in components of the RAS-signaling pathways. Instead, sotorasib-resistant tumors displayed amplification of the mutant Kras allele and activation of xenobiotic metabolism pathways, suggesting that reduction of the on-target activity of KRASG12C inhibitors is the main mechanism responsible for the onset of resistance. In sum, our results suggest that resistance to KRAS inhibitors could be prevented by achieving a more robust inhibition of KRAS signaling mimicking the results obtained upon Kras ablation.
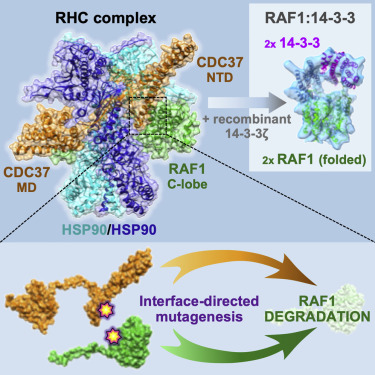
Structure of the RAF1-HSP90-CDC37 complex reveals the basis of RAF1 regulation
García-Alonso S, Mesa P, de la Puente Ovejero L, Aizpurua G, Lechuga CG, Zarzuela E, Santiveri CM, Sanclemente M, Muñoz J, Musteanu M, Campos-Olivas R, Martínez-Torrecuadrada J, Barbacid M, and Montoya G.
Abstract: RAF kinases are RAS-activated enzymes that initiate signaling through the MAPK cascade to control cellular proliferation, differentiation, and survival. Here, we describe the structure of the full-length RAF1 protein in complex with HSP90 and CDC37 obtained by cryoelectron microscopy. The reconstruction reveals a RAF1 kinase with an unfolded N-lobe separated from its C-lobe. The hydrophobic core of the N-lobe is trapped in the HSP90 dimer, while CDC37 wraps around the chaperone and interacts with the N- and C-lobes of the kinase. The structure indicates how CDC37 can discriminate between the different members of the RAF family. Our structural analysis also reveals that the folded RAF1 assembles with 14-3-3 dimers, suggesting that after folding RAF1 follows a similar activation as B-RAF. Finally, disruption of the interaction between CDC37 and the DFG segment of RAF1 unveils potential vulnerabilities in attempting the pharmacological degradation of RAF1 for therapeutic purposes.
Targeting the MAPK Pathway in KRAS-Driven Tumors
Drosten M and Barbacid M
Abstract: KRAS mutations occur in a quarter of all of human cancers, yet no selective drug has been approved to treat these tumors. Despite the recent development of drugs that block KRASG12C, the majority of KRAS oncoproteins remain undruggable. Here, we review recent efforts to validate individual components of the mitogenactivated protein kinase (MAPK) pathway as targets to treat KRAS-mutant cancers by comparing genetic information derived from experimental mouse models of KRAS-driven lung and pancreatic tumors with the outcome of selective MAPK inhibitors in clinical trials. We also review the potential of RAF1 as a key target to block KRAS-mutant cancers.
RAF1 kinase activity is dispensable for KRAS/p53 mutant lung tumor progression
Sanclemente M, Nieto P, García-Alonso S, Fernández-García F, Esteban-Burgos L, Guerra MC, Drosten M, Caleiras E, Martínez-Torrecuadrada J, Santamaría D, Musteanu M and Barbacid M
Complete Regression of Advanced Pancreatic Ductal Adenocarcinomas upon Combined Inhibition of EGFR and C-RAF»
Blasco MT, Navas C, Martín-Serrano G, Graña-Castro O, Lechuga CG, Martín-Díaz L, Djurec M, Li J, Morales-Cacho L, Esteban-Burgos L, Perales-Patón J, Bousquet-Mur E, Castellano E, Jacob HKC, Cabras L, Musteanu M, Drosten M, Ortega S, Mulero F, Sainz B Jr, Dusetti N, Iovanna J, Sánchez-Bueno F, Hidalgo M, Khiabanian H, Rabadán R, Al-Shahrour F, Guerra C, Barbacid M
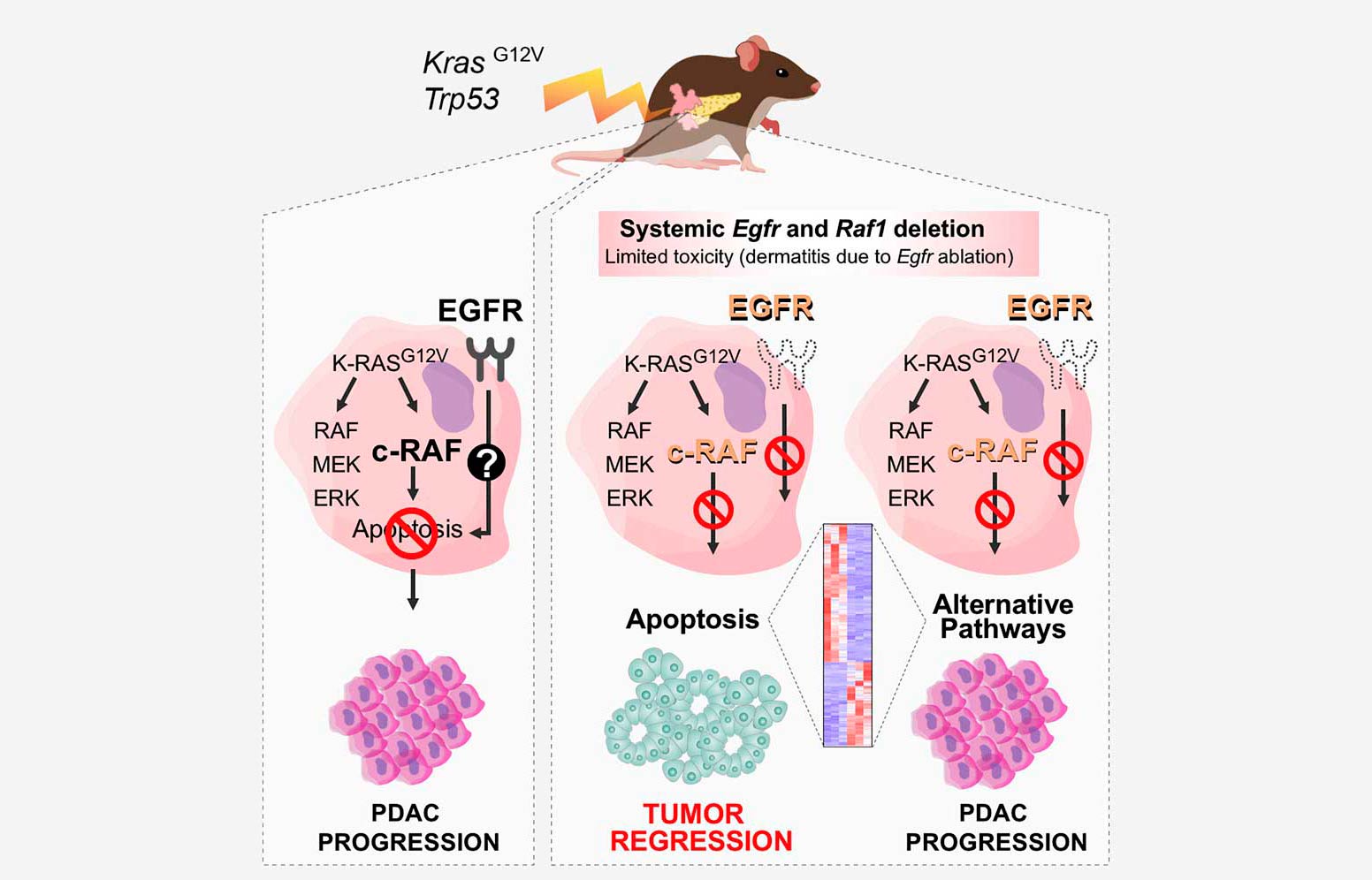
Abstract: Five-year survival for pancreatic ductal adenocarcinoma (PDAC) patients remains below 7% due to the lack of effective treatments. Here, we report that combined ablation of EGFR and c-RAF expression results in complete regression of a significant percentage of PDAC tumors driven by Kras/Trp53 mutations in genetically engineered mice. Moreover, systemic elimination of these targets induces toxicities that are well tolerated. Response to this targeted therapy correlates with transcriptional profiles that resemble those observed in human PDACs. Finally, inhibition of EGFR and c-RAF expression effectively blocked tumor progression in nine independent patient-derived xenografts carrying KRAS and TP53 mutations. These results open the door to the development of targeted therapies for PDAC patients.
c-RAF Ablation Induces Regression of Advanced Kras/Trp53 Mutant Lung Adenocarcinomas by a Mechanism Independent of MAPK Signaling»
Sanclemente M, Francoz S, Esteban-Burgos L, Bousquet-Mur E, Djurec M, Lopez-Casas PP, Hidalgo M, Guerra C, Drosten M, Musteanu M, Barbacid M
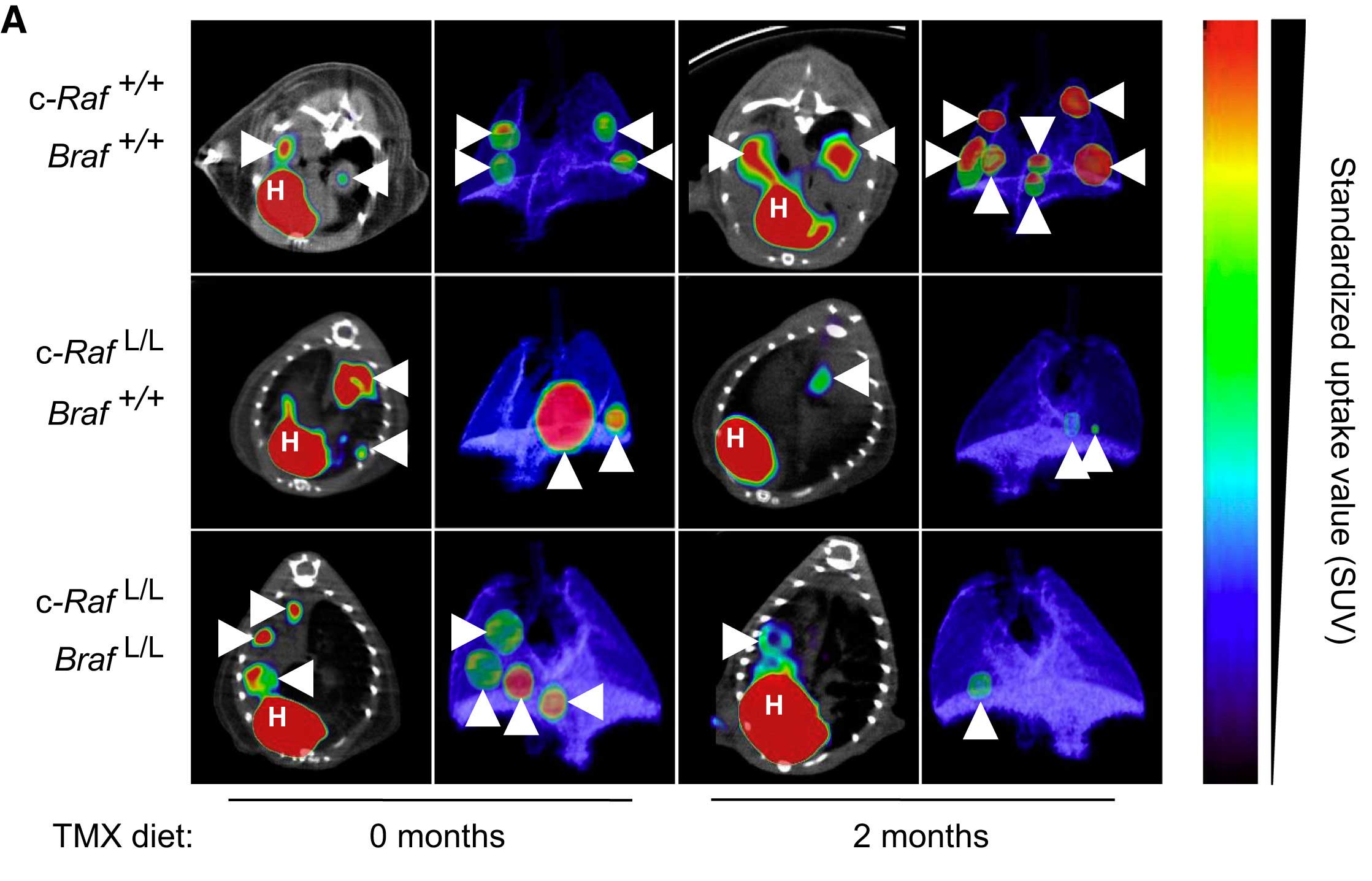
Abstract: A quarter of all solid tumors harbor KRAS oncogenes. Yet, no selective drugs have been approved to treat these malignancies. Genetic interrogation of the MAPK pathway revealed that systemic ablation of MEK or ERK kinases in adult mice prevent tumor development but are unacceptably toxic. Here, we demonstrate that ablation of c-RAF expression in advanced tumors driven by KrasG12V/Trp53 mutations leads to significant tumor regression with no detectable appearance of resistance mechanisms. Tumor regression results from massive apoptosis. Importantly, systemic abrogation of c-RAF expression does not inhibit canonical MAPK signaling, hence, resulting in limited toxicities. These results are of significant relevance for the design of therapeutic strategies to treat K-RAS mutant cancers.
Loss of p53 induces cell proliferation via Ras-independent activation of the Raf/Mek/Erk signaling pathway»
Drosten M, Sum EY, Lechuga CG, Simón-Carrasco L, Jacob HK, García-Medina R, Huang S, Beijersbergen RL, Bernards R, Barbacid M
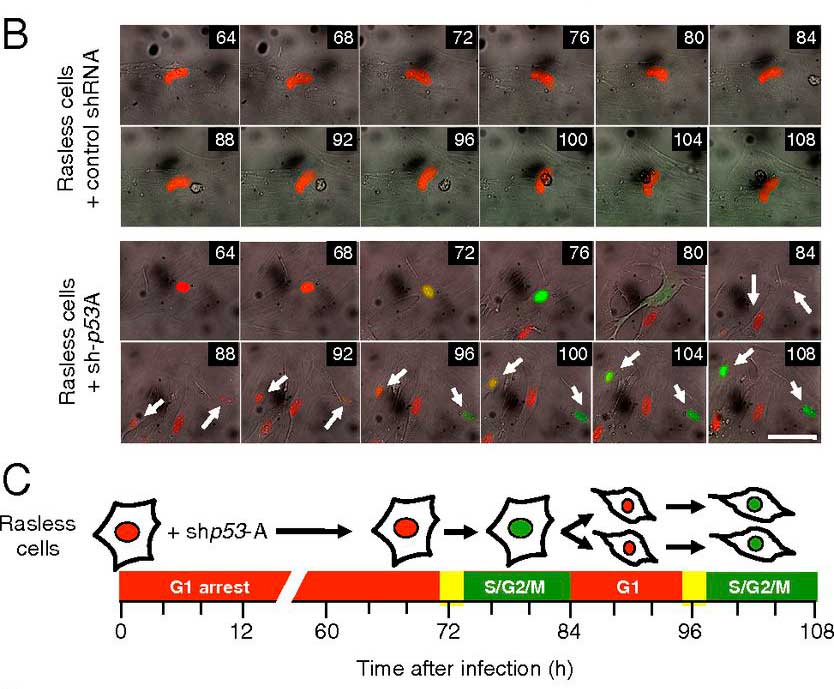
Abstract: The Ras family of small GTPases constitutes a central node in the transmission of mitogenic stimuli to the cell cycle machinery. The ultimate receptor of these mitogenic signals is the retinoblastoma (Rb) family of pocket proteins, whose inactivation is a required step to license cell proliferation. However, little is known regarding the molecular events that connect Ras signaling with the cell cycle. Here, we provide genetic evidence to illustrate that the p53/p21 Cdk-interacting protein 1 (Cip1)/Rb axis is an essential component of the Ras signaling pathway. Indeed, knockdown of p53, p21Cip1, or Rb restores proliferative properties in cells arrested by ablation of the three Ras loci, H-, N- and K-Ras. Ras signaling selectively inactivates p53-mediated induction of p21Cip1 expression by inhibiting acetylation of specific lysine residues in the p53 DNA binding domain. Proliferation of cells lacking both Ras proteins and p53 can be prevented by reexpression of the human p53 ortholog, provided that it retains an active DNA binding domain and an intact lysine residue at position 164. These results unveil a previously unidentified role for p53 in preventing cell proliferation under unfavorable mitogenic conditions. Moreover, we provide evidence that cells lacking Ras and p53 proteins owe their proliferative properties to the unexpected retroactivation of the Raf/Mek/Erk cascade by a Ras-independent mechanism.




